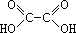|
By Kimberly Page High levels of the toxin commonly known as oxalic acid cause oxalate poisoning. Oxalic acid is produced naturally in the body when ascorbic acid and glycine are metabolized. These low levels formed are excreted in the urine in measurable amounts. Oxalic acid is found in low and high levels in certain fruits and vegetables that are common in some diets today. Low levels of oxalic acid are found in tomatoes, grapes, and sweet potatoes. High levels of the acid are found in spinach, sorrel, and rhubarb (Sanz 1992). The focus of this research centered on the levels in rhubarb and the effects they cause on the human body.
In the 18th century it was first reported of the petioles being used for food in England. During this time the leaves of the rhubarb plant were used as a table green, much like spinach is today. However, at this time it was not yet understood that the toxin is located in the leaves and can be fatal if high levels are consumed. Death was, and is still, rare due to consumption of the rhubarb leaves.
In the 19th century it was introduced to the United States. It did not gain the popularity in the United States as it had in Europe. At this time botanist and horticulturist did not agree on the taxonomy of the crop. Due to the hybrid versions produced, which came to be known as mule varieties, there was an uncertainty as to the species origin of that used for cooking (Thirsk 1995).
The stalk has been used as a laxative drug and a general tonic tracing back to ancient China. Today it is being used as part of a treatment for Aids patients though there is no scientific evidence supporting its use. This formula used is Essiac. In one reported case the patient involved lost 20 pounds due to diarrhea (http://www.primenet.com/~camilla/essiac. Today’s research of Essiac has confirmed what the ancient Chinese knew, that rhubarb contains a laxative agent. Today, there are some persons, such as in scotland that use the leaves in making a tea, even though as mentioned, high levels can be toxic. The tea is bitter to taste because of the leaves containing the oxalic acid. But this seems to be refreshing for those persons who have suffered a heat stroke or fever patients.
Cerebral edema is frequently found in the brain. Calcium oxalate crystals may be found in many vasa walls along with areas of focal necrosis and images of sterile meningitis with infiltrated neutrophils and mononuclear cells. In animals that eat oxalate-containing plants, macroscopic pathology reveals edema and hemorrhaging in the rumen wall with hyperemia of mucoses an ascites. Microscopic examination reveals calcium oxalate crystals in the rumen wall and in the renal tubules as well as microhemorrhaging in the medulla oblongat (Sanz 1992). Some persons are more prone to the development of the stones. It has been proposed that 3 or 4 genes are involved and influence the transport of the oxalic acid in the intestine or kidney (Goodman 1997). The binding of the calcium with the oxalic acids also lowers the levels of available calcium in the body (Weaver 1997). The loss of calcium interferes with electrical activity of the heart, muscles, and nerves, which explains the symptoms discussed after exposure to the toxin. The calcium depletion inhibits the action of the calcium pump that is involved in the action potential of the muscles. The calcium pump transports calcium from the cytosol to the lumen of the sarcoplasmic endoplasmic reticulum establishing a concentration gradient. When calcium channels open, calcium floods out of the sarcoplasmic/endoplasmic reticulum that allows for muscle contraction. The calcium pump transports calcium back into the lumen, which facilitate relaxation http://www.bio.davidson.edu/Biology/student/pumpmodel.html). Therefore, with the oxalic acid in the system binding with the calcium and depleting the available levels, the amount of calcium in the cytosol is reduced and leads to a continual state of relaxation of the muscles. Within smooth muscle, actin filaments are associated with a protein complex consisting of troponin and tropomyosin which covers the actin binding sites of the resting cell and which has an affinity for calcium. If calcium is present it associates with the troponin exposing the actin-binding site leading to muscle contraction. When calcium is resequestered, the binding sites are covered which leads to muscle relaxation. So again, when the amount of available calcium is depleted the actin binding sites remain covered and muscle relaxation persists. This phenomenon explains the CNS depression and cardiovascular collapse as described above. The loss of available calcium also effects the cardiac output of the heart. The lack of calcium leads to heart stoppage. However, the stomach has been proven helpful in the absorption of oxalate and also is critical for intestinal oxalate absorption in an intact gastrointestinal tract. Oxalate absorption appears to occur along the course of the entire gastrointestinal tract. The studies have shown that persons with an intact intestinal tract, urinary oxalate increased promptly when they were challenged with the gastric oxalate load that was subsequently hindered form travel through the intestinal tract (Hautmann 1993) Sodium oxalate is considered highly toxic through all routes of exposure. In a case report of accidental oxalate poisoning, a 16-year-old girl received an injection of 1.2 g of sodium oxalate during hospital treatment. Clinical death occurred within 5 minutes and although cardiac massage restored cardiac activity, the patient remained in a coma until death four days later. It has been concluded that the relative toxicity of oxalic acid and sodium oxalate appears to be about equal considering the differences in molecular weight (Anonymous 1994). Being exposed to the toxin may also result in hyperoxaluria which is characterized by nephrocalcinosis and nephrolithiasis. There are two types. The prinary type is a rare autosomal recessive disorder with excessive calcium oxalate synthesis and urinary excretion. The secondary type develops as a result of intoxication by ethylene glycol, the anaesthetic methoxyflurane, excessive intake of oxalic acid and xylitol, pyridoxine deficiency, and diseases involving the terminal ileum. Oxalosis is the deposition of calcium oxalate crystals in extrarenal tissues including bone, bone marrow, heart, blood vessels, central nervous system, peripheral nerves, liver, spleen, thyroid and adrenals. Renal abnormalities are generally the first and main manifestations of the condition, with organs being affected later. Renal failure and death eventually develop as a result of severe interstitial nephritis secondary to oxalate deposits (Akhan 1995).
A bacteria Pseudomonas oxalaticus is a bacteria identified as capable of metaboliing oxalates to carbon dioxide and water. These bacteria are present in humans also. The bacteria are important in the prevention of excess oxalate absorption. Oxalate added to active sludge appears to have a degradation half-life in the range of 14 days. Oxalic acid reacts with a number of cations to form soluble as well as insoluble salts. Heavy metals pose a concern because of their potential hazardous nature and possible adverse effects on human health and the environment. Interactions of the metals with oxalic acid or cationic exchange with oxalates could allow the migration and accumulation of the heavy metals through the soil that causes concern for the water supplies. Oxalic Acid is a powerful wood
cleaner, it is ideal for cleaning grey or blackened timber caused by
weathering. It takes out the grey dirt and kills any mould ,bringing the
surface to the rich look of new wood. Oxalic Acid is ideal for cleaning
grey or blackened fences, decks and other exterior wood work. It can be
used on all types of timber and is especially useful for cleaning
blackened and weathered Western Red Cedar weatherboards. Timber which has
been previously coated with Linseed Oil or some other similar finish, may
require vigorous scrubbing with Oxalic acid to clean off the
discoloration.. References:
|